Your Content Goes Here
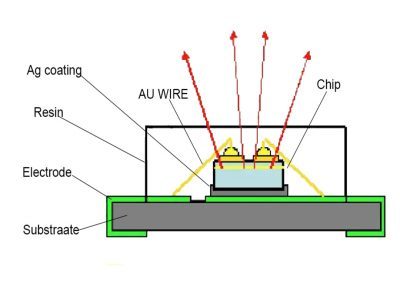
According to big data, there may be more than 100 reasons for LED dead lights. Today, we only take LED light source as an example to introduce some possible reasons for LED dead lights from the five raw materials of LED light source (gold wire, chip, bracket, phosphor, solid crystal adhesive and packaging adhesive).
Golden thread
- Copper alloy, gold clad silver alloy wire, silver alloy wire instead of gold wire
Gold wire has the advantages of high conductivity, good thermal conductivity, corrosion resistance, good toughness and excellent chemical stability. However, the price of gold wire is expensive, resulting in high packaging cost. In the periodic table of elements, gold, silver, copper and aluminum among the transition metal elements have high conductivity. Many LED manufacturers try to develop copper alloy, gold clad silver alloy wire and silver alloy wire to replace expensive gold wire. Although these alternatives are superior to gold wire in some properties, they are much worse in chemical stability. For example, silver wire and gold-clad silver alloy wire are vulnerable to sulfur / chlorine / bromination corrosion, and copper wire is prone to oxidation. For the encapsulated silica gel similar to the water absorbing and breathable sponge, these alternatives make the bonding wire vulnerable to chemical corrosion, reduce the reliability of the light source, and the LED lamp bead is easy to be disconnected and dead for a long time.
- Diameter deviation
1g gold, can be drawn with a length of 26.37m and a diameter of 50 μ M (2 mil) gold wire can also be drawn with a length of 105.49m and a diameter of 25 μ M (1 mil) gold wire. If the length of the gold wire is fixed, and if the diameter of the incoming gold wire is half of the original, the resistance measured for the gold wire is one fourth of the normal.
For suppliers, the thinner the diameter of the gold wire, the lower the cost, and the higher the profit when the selling price remains the same. For LED customers who use gold wires, there is a risk that the resistance of gold wires will increase and the fusing current will decrease when purchasing gold wires with Jerry built diameters, which will greatly reduce the service life of LED light sources. For example, the life of 1.0 mil gold wire must be shorter than that of 1.2 mil gold wire.
- Surface defect
(1) The wire surface shall be free of nicks, dents, scratches, cracks, bulges, discounts and other defects that reduce the service life of devices exceeding 5% of the wire diameter. During the drawing process of gold wire, the surface defects on the wire surface will increase the current density, make the damaged parts easy to be burned, and reduce the ability to resist mechanical stress, resulting in the fracture of the damaged part of the inner lead.
(2) The surface of the gold wire shall be free of oil, rust, dust and other adherents, which will reduce the bonding strength between the gold wire and the LED chip, and between the gold wire and the bracket.
- Too low breaking load and elongation
A good gold wire that can withstand the impact produced by resin packaging must have the specified breaking load and elongation. At the same time, the breaking force and elongation of gold wire play a key role in the quality of wire bonding, and the bonding wire with high breaking force and elongation is more conducive to bonding. Too soft gold wire will cause the following defects:
(1) Arch wire sagging;
(2) Spherical instability;
(3) The neck of the ball is easy to contract;
(4) Gold thread is easy to break.
Too hard gold wire will cause the following defects:
(1) Hole the chip electrode or epitaxy;
(2) The neck of Golden Globe is broken;
(3) Difficult to form alloy;
(4) Arch wire arc control is difficult.
chip
- Poor antistatic ability of chip
The antistatic index of LED lamp beads depends on the LED light-emitting chip itself, and is basically irrelevant to the expected packaging process of packaging materials, or the influencing factors are very small and subtle; LED lamps are more vulnerable to electrostatic damage, which is related to the distance between the two pins. The distance between the two electrodes of the bare crystal of the LED chip is very small, usually within 100 microns, while the distance between the LED pins is about 2 mm. When the electrostatic charge needs to be transferred, the greater the distance, the easier it is to form a large potential difference, that is, a high voltage. Therefore, electrostatic damage accidents are more likely to occur after sealing LED lamps.
- Chip epitaxial defect
During the high-temperature growth process of LED epitaxial wafers, the residual sediments in the substrate, MOCVD reaction chamber, peripheral gas and Mo source will introduce impurities, which will penetrate into the epitaxial layer, prevent gallium nitride crystal nucleation, form various epitaxial defects, and finally form tiny pits on the surface of the epitaxial layer, which will also seriously affect the crystal quality and performance of epitaxial film materials.
- Chip chemical residue
Electrode processing is a key process for making LED chips, including cleaning, evaporation, yellow light, chemical etching, fusion and grinding. It will be exposed to many chemical cleaning agents. If the chips are not cleaned thoroughly, harmful chemicals will remain. These harmful chemicals will react electrochemically with the electrode when the LED is powered on, resulting in dead light, light failure, dark light, blackening and other phenomena. Therefore, the identification of chip chemical residues is very important for LED packaging plants.
- Chip damage
The damage of LED display chip will directly lead to LED failure, so it is very important to improve the reliability of LED chip. During the evaporation process, sometimes a spring clip is used to fix the chip, so the clip mark will be produced. In yellow light operation, if the development is incomplete and the mask has holes, there will be residual excess metal in the luminous area. In the previous process, tweezers, flower baskets, vehicles, etc. must be used in various processes such as cleaning, evaporation, yellow light, chemical etching, fusion, grinding, etc., so the grain electrode may be scratched.
Influence of chip electrode on solder joint: the chip electrode itself is not reliably evaporated, resulting in electrode falling off or damage after welding wire; Poor solderability of chip electrode will lead to solder ball solder failure; Improper chip storage will lead to electrode surface oxidation, surface contamination and so on. Slight contamination of bonding surface may affect the diffusion of metal atoms between the two, resulting in failure or faulty soldering.
- Incompatibility between chips with new structure and light source materials
There is a layer of aluminum in the electrode of the LED chip with the new structure. Its function is to form a layer of reflector in the electrode to improve the light output efficiency of the chip. Secondly, it can reduce the use of gold during evaporation of the electrode to a certain extent, thereby reducing the cost. However, aluminum is a relatively active metal. Once the packaging plant fails to control the incoming materials and uses glue containing excessive chlorine, the aluminum reflector in the gold electrode will react with the chlorine in the glue, resulting in corrosion.
LED bracket
- Too thin silver coating
The existing LED light sources in the market choose copper as the base material of the lead frame. In order to prevent copper oxidation, a layer of silver should be plated on the surface of the bracket. If the silver plating layer is too thin, the bracket will turn yellow under high temperature. The yellowing of silver coating is not caused by the silver coating itself, but by the copper layer under the silver layer. At high temperature, copper atoms will diffuse and penetrate into the surface of the silver layer, making the silver layer yellow. The oxidizability of copper is the biggest drawback of copper itself. Once copper is oxidized, its thermal conductivity and heat dissipation performance will be greatly reduced. So the thickness of silver plating layer is very important. At the same time, both copper and silver are easily corroded by various volatile sulfide, halide and other pollutants in the air, making their surfaces dark and discolored. Some studies have shown that the color change increases the surface resistance by about 20 ~ 80% and the power loss, which greatly reduces the stability and reliability of LED and even leads to serious accidents.
- Silver coating vulcanization
The LED light source is afraid of sulfur because the sulfur-containing gas will react with the silver plating layer of the light source through the porous silica gel or bracket gap. After the vulcanization reaction of LED light source, the product functional area will blacken, the luminous flux will gradually decline, and the color temperature will drift obviously; After vulcanization, the conductivity of silver sulfide increases with the increase of temperature, which is easy to cause electric leakage in the process of use; The more serious condition is that the silver layer is completely corroded and the copper layer is exposed. As the gold wire 2 solder joint is attached to the surface of the silver layer, when the silver layer in the functional area of the support is completely vulcanized and corroded, the gold ball falls off, resulting in a dead lamp.
- Oxidation of silver coating
In the case of preliminary diagnosis of LED blackening, it is found that sulfur / chlorine / bromine is more difficult to find. However, the silver coating of LED light source shows obvious signs of blackening, which may be related to silver oxidation. However, pure element analysis and detection methods such as EDS energy spectrum analysis are not easy to judge oxidation, because the oxygen element existing in the air environment, sample surface adsorption, packaging glue and other organic substances will interfere with the determination of test results. Therefore, the conclusion of judging oxidation blackening requires the use of professional tests such as SEM, EDS, micro infrared spectroscopy, XPS and a series of reliability comparison experiments such as optical, electrical, chemical and environmental aging, Combined with professional testing knowledge and electroplating knowledge for comprehensive analysis.
- Poor electroplating quality
The quality of the coating mainly depends on the crystalline structure of the metal deposition layer. Generally speaking, the finer the crystalline structure, the denser and smoother the coating, and the higher the protective performance. This fine crystalline coating is called « microcrystalline deposit ». A good electroplating layer shall be fine, smooth, uniform and continuous, and shall not have any defects such as pollutants, chemical residues, spots, black spots, scorching, roughness, pinholes, pitting, cracks, delamination, blistering, peeling and wrinkling, peeling, yellowing, crystalline coating, local absence of coating, etc.
In electroplating practice, the thickness of metal coating and the uniformity and integrity of the coating are one of the important indicators to check the coating quality, because the protective performance and porosity of the coating are directly related to the coating thickness. The special change is the cathode coating. With the increase of the thickness, the protective performance of the coating is also improved. If the thickness of the coating is uneven, the thinnest part is often damaged first, and the coating on other parts will lose its protective effect no matter how thick it is.
The porosity of the coating is large, and corrosive gases such as oxygen will enter the corrosive copper matrix through the pores
- Organic pollution
Because various potions containing organic substances are used in the electroplating process, if the silver plating layer is not cleaned cleanly or the potions with poor quality and deterioration are selected, once these residual organic substances are in the environment where the light source is lit, under the action of light, heat and electricity, the organic substances may undergo oxidation-reduction and other chemical reactions, resulting in the discoloration of the silver plating surface.
- Nozzle material
The material of plastic is the key to the heat conduction of LED packaging support. If the PPA support is made of nozzle material, the plastic performance of PPA will be reduced, resulting in the following problems: poor high temperature bearing capacity, easy deformation, yellowing and low reflectivity; With high water absorption, the support will change in size and decrease in mechanical strength due to water absorption; Poor adhesion with metal and silica gel. It is not compatible with many silica gels. These potential problems make it difficult for the lamp beads to be used at slightly higher power. Once the power range is exceeded, the initial brightness is very high, but the attenuation is very fast, and the lamp will be dark within a few months.
Phosphor
- Phosphor hydrolysis
Nitride phosphors are easy to hydrolyze and fail.
- Mechanism of self heating of phosphors
Due to the self heating mechanism of phosphor, the temperature of phosphor layer is often higher than that of LED chip p-n junction. The reason is that the conversion efficiency of the phosphor can not reach 100%, so part of the blue light absorbed by the phosphor is converted into yellow light, and the other part of the light energy absorbed by the phosphor in the high light energy density LED package is turned into heat. Since the phosphor is usually mixed with silica gel, and the thermal conductivity of silica gel is very low, only 0.16 w/mk, the heat generated by phosphor will accumulate in a small local area, resulting in local high temperature. The greater the optical density of LED, the greater the heat generation of phosphor. When the temperature of the phosphor reaches more than 450 ℃, the silica gel near the phosphor particles will carbonize. Once the silica gel in a certain place is carbonized and blackened, its light conversion efficiency will be lower. This area will absorb more light energy from LED and convert more heat. The temperature will continue to increase, making the carbonization area larger and larger.
Solid crystal adhesive
- Silver glue stripping
The matrix of conductive silver adhesive is epoxy resin material, and its thermal expansion coefficient is much larger than that of chip and bracket. In the cold and hot impact environment of lamp beads, stress will be generated due to thermal problems, and the effect will be more intensified in the environment with drastic temperature changes. The colloid itself has tensile fracture strength and ductility. When the tensile force exceeds, the colloid will crack. The solid crystal adhesive is peeled off at the interface, the heat dissipation becomes worse sharply, the heat generated by the chip cannot be exported, and the junction temperature rises rapidly, which greatly accelerates the process of light decay.
- Silver glue delamination
Silver powder particles are dispersed in the slurry system in a suspended state. Due to the influence of many factors such as density difference, charge, cohesion, force and the structure of the dispersion system between the silver powder and the matrix, the phenomenon of silver powder sedimentation and stratification often occurs. If the sedimentation is too fast, the product will have sagging during slurry hanging, the coating thickness will be uneven, and even affect the physicochemical properties of the coating film. Stratification will also affect the heat dissipation Bonding strength and conductivity.
- Silver ion migration
A customer’s vertical flip-flop light source sealed with silica gel and bonded with conductive silver glue has leakage. Through the analysis of defective light beads. Abnormal silver elements were detected on the side of the chip, and it was observed that the silver particles gradually spread from the bottom positive silver glue area to the side of the p-n junction on the top of the chip in a dendritic shape. Therefore, it was determined that the leakage failure of the defective lamp beads was most likely caused by the migration of silver ions from the solid crystal silver glue on the side of the chip. The phenomenon of silver ion migration is gradually formed during the use of the product. With the aggravation of the migration phenomenon, the silver ion will eventually conduct the chip p-n junction, resulting in low resistance path on the side of the chip, resulting in abnormal leakage current of the chip, and even short circuit of the chip in serious cases. There are many reasons for the migration of silver, but the main reason is that the silver based material is damped. After the silver glue is damped, the invading water molecules ionize the silver and migrate along the side of the chip under the action of the vertical electric field from bottom to top. Therefore, it is recommended that the customer should carefully use silica gel packaging and silver glue to bond the lamp beads of the vertical flip chip, select the Gold Tin eutectic welding method to fix the chip on the bracket, and strengthen the waterproof characteristic detection of the lamp.
- Solid crystal adhesive does not dry
The curing agent of silicone used for LED packaging contains platinum (Platinum) complex, which is very easy to be poisoned. The toxic agent is any compound containing nitrogen (n), phosphorus (P) and sulfur (s). Once the curing agent is poisoned, the silicone curing is incomplete, resulting in high linear expansion coefficient and increased stress.
Sealant
- Poor heat resistance of glue
According to our test results, pure silica gel began to crack at 400 ℃, but the heat resistance of modified silica gel added with epoxy resin was reduced to the level of epoxy resin. When this modified silica gel is applied to high-power LED or high-temperature environment, the colloid will be yellow, black, cracked and dead.
- Glue does not dry
The curing agent of silicone used for LED packaging contains platinum (Platinum) complex, which is very easy to be poisoned. The toxic agent is any compound containing nitrogen (n), phosphorus (P) and sulfur (s). Once the curing agent is poisoned, the silicone curing is incomplete, resulting in high linear expansion coefficient and increased stress.
Substances prone to silica gel « poisoning » include: organic compounds containing N, P, s, etc; Sn, Pb, Hg, Sb, Bi, as and other heavy metal ionic compounds; Organic compounds containing unsaturated groups such as ethynyl. Pay attention to the following materials:
Organic rubber: sulfur vulcanized rubber such as gloves
Epoxy resin, polyurethane resin: amine, isocyanate resin curing agent
Comprehensive silicone RTV rubber: especially Sn catalyst
Soft cyanoethylene: plasticizer and stabilizer
Flux
Engineering Plastics: flame retardant, heat-resisting agent, ultraviolet absorber, etc
Silver plated and gold plated surfaces (the electroplating solution during manufacturing is the main reason)
Degassing produced by solder register (caused by heating and curing of silicone)
- The linear expansion coefficient of packaging adhesive is too large
In the cold and hot impact environment of lamp beads, stress will be generated due to thermal problems, and the effect will be more aggravated in the environment with violent temperature changes. The colloid itself has tensile breaking strength and ductility. When the tensile force exceeds, the colloid will crack. 4. Glue contains chlorine
However, at present, domestic epoxy resin manufacturers generally have small production scale, backward management mode and production process, and low automation of operation machinery, which makes it difficult to guarantee the parameters of epoxy resin. The production of low-quality epoxy resin is related to the current industrial situation in China, and the industry is in urgent need of upgrading.
The chlorine in the epoxy resin not only causes chlorination corrosion to the silver coating of the support, alloy wire or other active metal and chip electrode (aluminum reflector), but also affects the curing of the resin by complexing with amine curing agent. Chlorine content is an important physical property index of epoxy resin. It refers to the mass fraction of chlorine in epoxy resin, including organic chlorine and inorganic chlorine. Inorganic chlorine will affect the electrical properties of the cured resin. The content of organic chlorine indicates the content of the chlorohydrin group in the molecule that does not have closed-loop reaction. Its content should be reduced as much as possible, otherwise it will also affect the curing of the resin and the performance of the cured product.